Asibe Özkan 1, Esra Çömezoğlu 2
1 Department of Obstetrics and Women’s Health Nursing, University of Health Sciences, Hamidiye Faculty of Nursing, Istanbul, Türkiye, ORCID: 0000-0002-4278-5278. asibeozkan@gmail.com
2 Department of Midwifery, Eskisehir Osmangazi University Health Sciences Institute, Eskisehir, Türkiye, ORCID: 0000-0002-6102-0078.
Received: 25 March 2024
Revised: 28 March 2024
Accepted: 28 March 2024
Published: 28 March 2024
ABSTRACT
| Venous thromboembolism (VTE) is a leading cause of maternal morbidity, so caution is required for prevention and rapid treatment. Early markers of the diagnosis of VTE during pregnancy are extremely important. We aimed to investigate the value of new inflammatory markers, neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte (PLR), in pregnant women with suspected deep vein thrombosis (DVT). In a single tertiary care center, pregnant women who were admitted to the emergency department and the outpatient clinics between January 2017 and December 2020 with the suspicion of DVT were determined retrospectively. NLR and PLR were compared between patients with and without DVT. Statistical analysis was performed using IBM SPSS Statistics 22 software. In our analyses, both NLR and PLR were significantly higher in patients with DVT. NLR and PLR were found to be higher in patients with DVT in the first trimester. The AUC value of the ROC curve of the NLR for the presence of DVT was 0.606; the NLR value was 3.74, the sensitivity was 60.5% and the specificity was 60.9%. The AUC of the ROC curve of the PLR for the presence of DVT was 0.667; the sensitivity of the PLR 142.6 was 62.1% and the specificity was 61.7%. During antenatal care, complete blood counts are routinely practiced per guideline recommendations in pregnant women, and close monitoring of NLR and PLR values in pregnant women with symptoms of DVT can prompt the midwives and obstetric nurses to accelerate the diagnosis of DVT by referring to a physician. |
Keywords:
Venous thromboembolism, deep vein thrombosis neutrophil to lymphocyte ratio, NLR, platelet to lymphocyte ratio, PLR.
Cite as: Özkan A, Çömezoğlu E. The Utility of Neutrophil to Lymphocyte and Platelet to Lymphocyte Ratios in Pregnant Women with DVT. Acta Med Eur. 2024;6(2):46-49. doi: 10.5281/zenodo.10889295
INTRODUCTION
Deep vein thrombosis (DVT) is observed at a rate of 100-200/100.000 in the general population and is one of the most frequent cardiovascular diseases along with myocardial infarction and stroke. Associated with serious mortality and mortality (1,2). DVT can lead to severe morbidity including pulmonary embolism, post-phlebitic syndrome, and pelvic congestion syndrome. Venous thromboembolism (VTE) is a leading cause of maternal morbidity, therefore, requires vigilance for prevention and prompttreatment (3,4).
Thrombophilia and hypercoagulable states predispose patients to venous thromboembolism (VTE), while it can be seen in patients of all age groups regardless of comorbidities. Young women are at increased risk due to the use of oral contraceptives or pregnancy. Diagnosis of VTE during pregnancy is especially important and can be more difficult than in other patient populations.Leg swelling, lower extremity edema, and pain are often present with the growing uterus exerting pressure on the pelvic veins. WHO recommends a continuous care model led by midwives or obstetric nurses that treats the woman as a whole and provides continuous follow-up in the intrapartum, antenatal and postpartum periods (5). Antenatal care is an important opportunity for early and rapid diagnosis of DVT and prevention of its complications. The awareness of midwives and nurses about the signs and findings associated with DVT is important for a prompt and correct diagnosis of DVT in the antenatal period, and thereby the prevention ofrelated morbidity and maternal mortality
The role of inflammation in the pathophysiology of venous thromboembolism is acknowledgedas a key risk factor (6,7). The neutrophil-to-lymphocyte (NLR) and platelet-to-lymphocyte ratios (PLR) are novel inflammatory biomarkers that are associated with disease activity and severity in cardiovascular diseases (8,9). These new markers may have value during the initial diagnostic work-up of pregnant women with suspicion of DVT in the emergency setting (10,11).
The value of the novel inflammatory markers, NLR and PLR, in pregnant women in the diagnostic process of DVT is unknown. We aimed to investigate the value of NLR and PLR in pregnant women with suspicion of DVT.
METHODS
In a single tertiary care center, pregnant women who were admitted to the emergency department and the outpatient clinics between January 2017 and December 2020 with the suspicion of DVT were retrospectively identified. Study Approval was obtained from the institutional academic board. Patients with signs and symptoms suggesting DVT and high D-Dimer were referred for Doppler ultrasonography (USG) within 24 hours. If the Doppler USG was negative for DVT and clinical suspicion of DVT persisted, a repeat Doppler USG was planned within 72 hours. Pulmonary Embolism (PE) was suspected if the patient had coexisting pulmonary symptoms or chest pain and was evaluated with echocardiography. Patient demographics, ultrasonography findings, and laboratory results were recorded. Blood count results were used to calculate NLR and PLR at the time of diagnosis. NLR and PLR were compared between patients with and without DVT. Patients with lower extremity DVT were grouped according to thrombus localization as denoted by the most proximal vein with thrombus. NLR and PLR were compared in patients with different thrombus localizations and the presence of PE.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 22 software. Nominal variables are presented as numbers and percentages and continuous variables are presented as mean and standard deviation. The Chi-squared test or Fisher’s exact test was applied for nominal variables, Student’s t-test for continuous parametric variables, and Mann Whitney U test for continuous non-parametric variables in group comparison. A p-value of < 0.05 was accepted as statistically significant.
RESULTS
During the study period, 205 pregnant women were referred to our hospitalwith suspicion of DVT. 124 of these patients were positive for DVT with a Doppler ultrasound and 81 patients did not have a venous thrombus. The mean age of all the study patients was 31.6 ± 5.6.Fourpatientswere in their third pregnancy, eight patients were in their second, and the remaining patients were in their first pregnancy. All patients had singleton pregnancies.
Patients were questioned for known thrombophilia disorders. Four patients had Factor V Leiden mutations, while two other patients had a history of prior thrombotic stroke and repeated DVTs but were not investigated for a definitive diagnosis.
The laterality of DVT was assessed in the study patients. In the majority of the patients (92, 74.1%) DVT was on the left lower leg and in six patients on the right leg. In twopatients, there were DVTs on both lower extremities, and one patient presented with DVT in the upper left extremity. Four patients with iliac DVT had echocardiographic findings consistent with pulmonary embolism at the time of diagnosis.
Patients with and without DVT were compared for patient factors and biochemical parameters (Table 1). Both NLR and PLR were significantly elevated in cases with DVT. Patients were grouped according to their trimester. Among patients in their first trimester, patients with DVT had higher NLR and PLR, while a difference could not be shown in patients in their second and third trimesters. The ROC curve of NLR for the presence of DVT had an AUC of 0.606, with an NLR of 3.74 having a sensitivity of 60.5% and specificity of 60.9%. The ROC curve of PLR for the presence of DVT had an AUC of 0.667, with a PLR of 142.6 having a sensitivity of 62.1% and specificity of 61.7% (Figure 1).
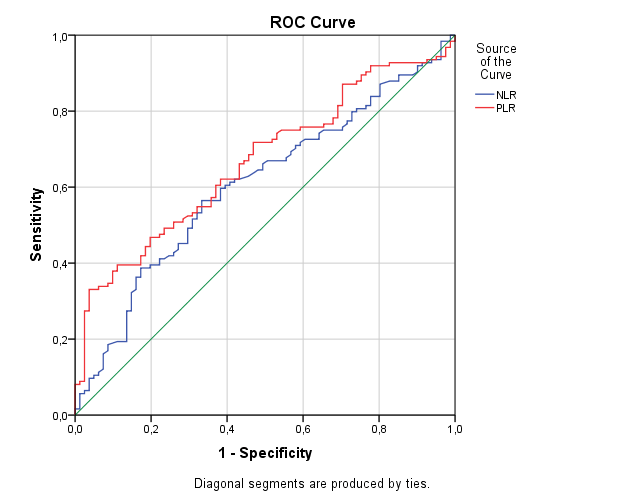
Figure 1. ROC Curve of NLR and PLR for the presence of DVT.
NLR and PLR were assessed with the localization of thrombus in the 123 patients with lower extremity DVT. NLR and PLR showed an increase with the higher level of DVT (Table 2). The ability of NLR and PLR in predicting the level of DVT was assessed using ROC curves. High level DVT was defined as iliac DVT with or without PE and low level DVT as DVT in the femoral or lower veins. For differentiating high and low level DVTs, NLR had an AUC of 0.681 while PLR had an AUC of 0.631. An NLR of 4.25 had a sensitivity of 72.0% and a specificity of 69.4%. A PLR of 173.4 had a sensitivity of 60.0% and a sensitivity of 64.3% (Figure 2).
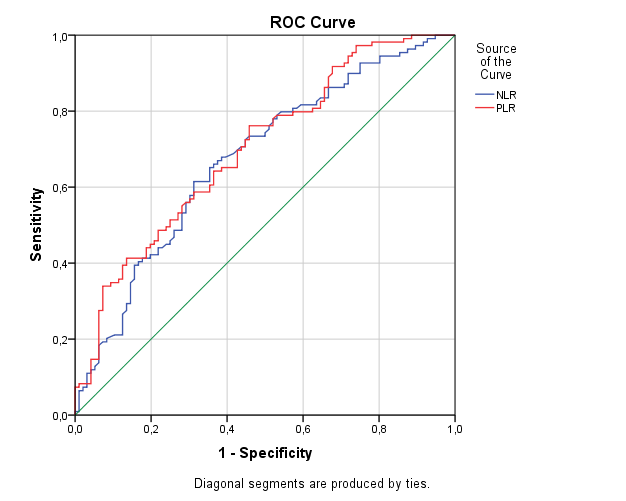
Figure 2. ROC Curve of NLR and PLR for high level DVT.
Table 1. Comparison between the groups in terms of patient factors and biochemical parameters.
| No DVT (n=81) | DVT (n=124) | p | |
| Age | 30.9 ± 5.3 | 32.0± 5.7 | 0.184 |
| Well’s Score | 2.37 ± 0.66 | 2.52± 0.62 | 0.100 |
| Known thrombophilia | 0 (0%) | 4 (3.23%) | |
| D-Dimer | 1177.9±155.26 | 1216.4± 192.67 | 0.134 |
| NLR | 3.46±1.09 | 3.84± 1.18 | 0.022 |
| PLR | 138.46± 24.58 | 147.83± 31.82 | 0.026 |
| Trimester | |||
| 1st | 20 (24.7%) | 34 (27.4%) | |
| NLR | 2.37 ± 0.83 | 3.62± 1.04 | <0.001 |
| PLR | 131.52±16.23 | 143.49± 21.72 | 0.037 |
| 2nd | 33 (40.7%) | 46 (37.1%) | |
| NLR | 3.83± 1.02 | 3.93± 0.89 | 0.645 |
| PLR | 142.29± 26.85 | 148.56 ± 48.40 | 0.503 |
| 3rd | 28 (34.6%) | 44 (35.5%) | |
| NLR | 3.68± 1.45 | 3.91± 1.29 | 0.485 |
| PLR | 134.89 ± 30.99 | 149.29± 47.57 | 0.160 |
NLR: Neutrophl-to-lymphocyte ratio, PLR: Plathelet-to-lymphocyte ratio.
DISCUSSION
Inflammatory markers of NLR and PLR may be useful during the diagnosis of DVT in pregnant women. These markers showed an increase inthe presence of DVT in pregnant women in our cohort. Both markers were more elevated with the higher level of DVT. High values of NLR and PLR should prompt caregivers of pregnant women with suspected DVT to refer for early imaging and treatment.
DVT in pregnancy is a significant cause of maternal mortality with venous thromboembolism (VTE) being a leading cause of maternal death in developed countries. Most VTE events during pregnancies are associated with silent DVTs. Pregnancy is associated with a 4-fold increase in the risk of DVT for women with hematologic changes and compression of the abdominal veins (12). DVT can present during the first pregnancy and regardless of the age of the mother. Edema and leg pain are often present in pregnant women due to compression of pelvic venous and lymphatic structures (13). Physical examination is less reliable than the general population when diagnosing DVT during pregnancy. A negative D-Dimer test rules out VTE during pregnancy as in non-pregnant patients, however, the levels of D-Dimer change during the course of the pregnancy, increasing over the gestational period(14,15). The use of trimester-specific D-Dimer may be more discriminatory but has not been incorporated into international guidelines (16,17).Together with other findings, high NLR or PLR can help the caregiver during the differential diagnosis of DVT.
Pregnancy is associated with a hypercoagulable state that is protective against excessive birth-related bleeding (18,19). The compression of the iliac veins by the growing uterus creates venous stasis that predisposes the patients to DVT as the pregnancy progresses.Contrary to the general population, proximal and left sided vein thrombosis is more common in pregnancy (20). Among our patients, iliofemoral DVT was more common than distal DVT and there was a greater tendency for left-sided DVT. Proximal DVT carries a higher risk of PE. A prompt diagnosis and initiation of treatment are important for the prevention of the mortality and morbidity associated with DVT.
The presence of DVT or PE is associated with increased inflammation that is reflected in the elevated inflammatory markers. The state of inflammation appears higher with the more proximal location of the thrombus which resulted in higher ratios. While higher than normal ratios are expected with DVT, levels of NLR and PLR vary naturally with the course of pregnancy (21,22). While NLR and PLR were evidently higher in pregnant women in their first trimester, the difference did not remain significant in patients in their second and third trimesters despite higher ratios seen with DVT. In this regard, further studies are needed to ascertain the diagnostic value of the inflammatory markers at different stages of pregnancy.
This was a single center, retrospective study, and data from other centers can help with external validation of our results. limiting the number of pregnant patients.The value of NLR and PLR in denoting DVT across different trimesters can be the focus of future studies that include more patients.
Table 2. NLR and PLR with different thrombus levels.
| N=123 | Poplitealand calf veins (n=34) | Femoral (n=64) | Iliac (n=21) | Iliac and PE (n=4) | p |
| NLR | 3.26± 1.48 | 3.90± 1.44 | 4.28± 1.16 | 5.42 ± 0.91 | 0.005 |
| PLR | 128.78± 46.70 | 148.58± 51.90 | 170.42± 53.00 | 179.28 ± 67.19 | 0.019 |
PE: Pulmonary embolism, NLR: Neutrophl-to-lymphocyte ratio, PLR: Plathelet-to-lymphocyte ratio.
DVT is one of the most important pathologies that needs early diagnosis and treatment. Leg complaints such as swelling and pain are common in pregnant women and additional findings that support differential diagnosis of DVT may help the diagnostic process. Our study shows that inflammatory markersare raised with the presence of DVT in pregnant womenand that higher levels of DVT is associated with increased NLR and PLR, reflecting a greater inflammatory activity. During antenatal care, complete blood counts are routinely practiced per guideline recommendations in pregnant women, and close monitoring of NLR and PLR values in pregnant women with symptoms of DVT can prompt the midwives and obstetric nurses to accelerate the diagnosis of DVT by referring to a physician.
Funding
This research received no external funding.
Conflict of Interest
The authors declares no conflict of interest.
REFERENCES
- Authors/Task Force Members, Konstantinides SV, Torbicki A, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–3080.
- Gülen ŞT. Pulmoner Tromboemboli: Tanım ve Epidemiyoloji. Turk Klin Pulm Med – Spec Top. 2016;9(1):1–5.
- James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006;194(5):1311–1315.
- Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-Related Mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130(2):366–373.
- World Health Organization. WHO recommendations on antenatal care for a positive pregnancy experience [Internet]. Geneva: World Health Organization; 2016 [cited 2022 Jun 28]. 152 p. Available from: https://apps.who.int/iris/handle/10665/250796
- Saghazadeh A, Rezaei N. Inflammation as a cause of venous thromboembolism. Crit Rev Oncol Hematol. 2016 Mar;99:272–285.
- Colling ME, Tourdot BE, Kanthi Y. Inflammation, Infection and Venous Thromboembolism. Circ Res. 2021;128(12):2017–2036.
- Artoni A, Abbattista M, Bucciarelli P, et al. Platelet to Lymphocyte Ratio and Neutrophil to Lymphocyte Ratio as Risk Factors for Venous Thrombosis. Clin Appl Thromb Off J Int Acad Clin Appl Thromb. 2018;24(5):808–814.
- Velioğlu Y, Yüksel A. Utility of platelet-to-lymphocyte ratio to support the diagnosis of acute deep vein thrombosis. Turk Gogus Kalp Damar Cerrahisi Derg. 2019;27(4):493–498.
- Rinaldi I, Hamonangan R, Azizi MS, et al. Diagnostic Value of Neutrophil Lymphocyte Ratio and D-Dimer as Biological Markers of Deep Vein Thrombosis in Patients Presenting with Unilateral Limb Edema. J Blood Med. 2021;12:313–325.
- Ming L, Jiang Z, Ma J, Wang Q, Wu F, Ping J. Platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio, and platelet indices in patients with acute deep vein thrombosis. VASA Z Gefasskrankheiten. 2018;47(2):143–147.
- Sousa Gomes M, Guimarães M, Montenegro N. Thrombolysis in pregnancy: a literature review. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2019;32(14):2418–2428.
- Devis P, Knuttinen MG. Deep venous thrombosis in pregnancy: incidence, pathogenesis and endovascular management. Cardiovasc Diagn Ther. 2017;7(Suppl 3):S309–S319.
- Bellesini M, Robert‐Ebadi H, Combescure C, Dedionigi C, Le Gal G, Righini M. D‐dimer to rule out venous thromboembolism during pregnancy: A systematic review and meta‐analysis. J Thromb Haemost. 2021 Oct 20;19(10):2454–67.
- Murphy N, Broadhurst D, Khashan A, Gilligan O, Kenny L, O’Donoghue K. Gestation-specific D-dimer reference ranges: a cross-sectional study. BJOG Int J Obstet Gynaecol. 2015;122(3):395–400.
- Parilla B, Fournogerakis R, Archer A, et al. Diagnosing Pulmonary Embolism in Pregnancy: Are Biomarkers and Clinical Predictive Models Useful? Am J Perinatol Rep. 2016;06(02):e160–164.
- Cohen SL, Feizullayeva C, McCandlish JA, et al. Comparison of international societal guidelines for the diagnosis of suspected pulmonary embolism during pregnancy. Lancet Haematol. 2020;7(3):e247–e258.
- Lockwood CJ. Pregnancy-associated changes in the hemostatic system. Clin Obstet Gynecol. 2006;49(4):836–843.
- Hellgren M. Hemostasis during normal pregnancy and puerperium. Semin Thromb Hemost. 2003;29(2):125–130.
- Chan WS, Spencer FA, Ginsbergm JS. Anatomic distribution of deep vein thrombosis in pregnancy. CMAJ Can Med Assoc J. 2010;182(7):657–660.
- Morisaki N, Piedvache A, Nagata C, et al. Maternal blood count parameters of chronic inflammation by gestational age and their associations with risk of preterm delivery in the Japan Environment and Children’s Study. Sci Rep. 2021;11(1):15522.
- Wang X, Zheng X, Yan J, et al. The Clinical Values of Afamin, Triglyceride and PLR in Predicting Risk of Gestational Diabetes During Early Pregnancy. Front Endocrinol. 2021;12:723650.
