Ferda Hosgorler 1, Erhan Caner Akkaya 1, Basar Koc 1, Rabia Ilgin 1, Servet Kizildag 2
1 Department of Physiology, Dokuz Eylül University School of Medicine, Izmir, Türkiye
2 College of Vocational School of Health Services, Dokuz Eylul University School of Medicine, Izmir, Türkiye
Received: 23 April 2024
Revised: 26 April 2024
Accepted: 26 April 2024
Published: 26 April 2024
ABSTRACT
| Naltrexone, a nonselective opioid receptor antagonist, is known to improve cognitive functions and exhibits neuromodulatory effects in the elderly. The aim of the study was to investigate the effects of Naltrexone on anxiety and empathy-like behavior in old rats. After learning period on empathy test, old rats subjected empathy-like behavior and anxiety-like behavior tests in two groups: Naltrexone-treated and non-Naltrexone treated. Corticosterone levels in serum were detected by ELISA. We observed that in anxiety tests (open field, elevated plus maze, corticosterone levels) of Naltrexone-treated rats showed less pronounced anxiety symptoms than non-Naltrexone treated rats. Naltrexone-treated rats showed better performance on the empathy-like behavior test. We surmised that Naltrexone improved helping behavior associated with anxiolytic effectiveness and can assume that stress is a factor which prevents helping behavior in aged subjects. |
Keywords:
Naltrexone, anxiety-like behavior, empathy-like behavior, corticosterone
Cite as:
INTRODUCTION
Empathy is comprised of emotional and cognitive skills and can be defined as the capacity to understand and respond to the emotional experiences of others. Empathy enables humans to establish social relationships and adapt to their community (1). Studies suggest that cognitive empathy decreases with advancing age (2-4). It has been suggested that cognitive empathy associated with the loss of attention, memory and perception, structural detoriorations in brain, slowing in process speed, and reduced reasoning ability may be diminished in the elderly (2, 3, 5). In our previous studies in rats, we observed helping behavior (empathy-like behavior), which may have exhibited a useful model for understanding the neurochemical processes of empathy (6-8).
Opioids are widely produced in the central nervous system. There are some records that opiods strengthen social bonds, reinforce social and affiliative behavior, and lead to altruistic behavior (9-11). Endogenous opioids have been implicated with anxiety-related behaviors. It has been reported that stressful stimuli increase kappa opioid receptor activity(12). Some studies suggest that kappa opioid receptor (KOR) blockers exhibit anxiolytic properties (13, 14).(15, 16). Naltrexone (NTX), a non-selective opioid receptor blocker (mu, delta and kappa receptors), can produce both reinforcing and repressive effects on behavior through different receptors (10). NTX improved aging-related impaired cognitive test scores in old rats and demonstrated neuroprotective and neuromodulatory effects in some neurological pathologies (such as behavioral addictions, chronic pain, experimental Parkinson model) (17-20).
The neuromodulatory effects of NTX may affect the helping behavior. The aim of this study was to investigate the effects of NTX on anxiety and empathy-like behavior in old rats.
METHODS
Experimental procedures in this study were carried out by our study team in accordance with the ‘Guide for the Care and Use of Laboratory Animals’ guide. Animals were obtained from the institutional animal laboratory and their care and procedures were approved in our institutional ethics committee (date and number: 05.04.2018-40/2018). All rats were fed with standard laboratory chow and kept in standard room temperature (22±1°C) and humidity (60%).
In this study, we used Sprague-Dawley male old rats (24-26 months, 390-430 g weight) divided into two groups. Control group: sham control rats (CR, n=8) received subcutanous injections of saline; treatment group: NTX-treated rats (NTR, n=8) received subcutanous injections of NTX. Both groups were subjected to the same experimental procedures.
For 30 days before the experiments, the rats were housed in the same cage with randomly selected pairs to get accustomed to one another. The rats were subjected to handling and then placed in the empathy apparatus for 7 days (5 minute) to enable them to adapt to the experimental environment. Following the adaptation period, for a duration of 12 days, all the rats were placed in the empathy apparatus for them to learn to rescue their cage mate.
An empathy-like behavior experiment was performed on the 13th day with saline (at a dose of 1 ml/kg) or NTX injections (at a dose of 1 mg/kg) subcutanously. After empathy-like behavior test other behavioral experiments were performed. Experiments were recorded using a video camera and a video tracking system device equipped with EthoVision XT Software (Noldus Information Technology Inc., Leesburg, VA, USA).
Immediately following empathy and other behavior experiments, all rats underwent thoracotomy under CO2 anesthesia and blood samples were obtained by right heart ventricule puncture. Blood samples were centrifuged at 1,000 g for 10 minutes and serums were collected and stored at -80 °C until the biochemical analysis.
Behavior Experiments
The empathy-like behavior experiment
In this study, an apparatus similar to the one described previously was used to test the empathy behavior in rats (21). The apparatus is a black plexiglass rectangular box (450x450x900 mm) separated in the middle by a transparent section which divides the box into two equal compartments; one of the compartments is a pool area and other is a dry area. The transparent part contains a transparent door which allows passage between the two areas. The observer rat in the dry compartment can open the door and rescue the cage mate, who is immersed in the pool compartment (Figure1a, 1b). The roles were reversed after the cage mate had dried. The experimental period was 300 seconds and the durations of rescue time for each rat was recorded.
The elevated plus-maze test
The elevated plus maze test is used to determine anxiety signs in rodents (22). In this test rodents were put on a platform which is above 50 cm from the ground and with a plus shape. The platform contains two open and two close arms which have 50 cm long. The rats explored the platform and choosed open or close arm to stay more time. The experiment duration was 300 seconds. We recorded staying duration at the arms and frequency of entry to the arms. Staying longer duration at the close arm than open arm or staying shorter duration at the open arm interprets as anxiety sign.
The open-field test
The open-field test is implemented to determine locomotor activity and anxiety of the rodents (23). In this test we utilized a black opaque plexiglass box (50x100x100 cm). The ground of the box was divided into equal squares (75% at the edges, 25% at the center). We left the rats at the center area of the box and recorded their exploration behavior for 300 seconds. We evaluated total distance travelled, time spent in the centre area and margin area in this test. The spending more time at the margin zone rather than the center zone indicates anxiety.
Biochemical measurement
ELISA measurement was performed according to the kit protocol to calculate serum corticosterone level. Rat specific Corticosterone ELISA Kit (Cat.No E0496Ra, Bioassay Technology Laboratory, China) was used. All results were calculated with mg protein per tissue. Absorbency changes were measured using a microplate reader (ELx800, BioTek Instruments, Inc., Winooski, Vt, U.S.A) at 450 nm for ELISA kits and at 560 nm for protein assay kit.
Statistical analysis
In the statistical evaluation of data was applied SPSS 24.0 software (IBM, Turkey). The Kolmogorov-Smirnov normality test was performed. The T-test analysis was applied when variables in normal distribition. When the variables were not normally distributed the Kruskal Wallis test was used. Correlations among the variables in normal distribition were calculated by Pearson correlation analysis. p values < 0.05 were considered statistically significant. Median and standard deviation values were indicated. The minimum, maximum, median and quartile values of each group were presented in box plot graphs.
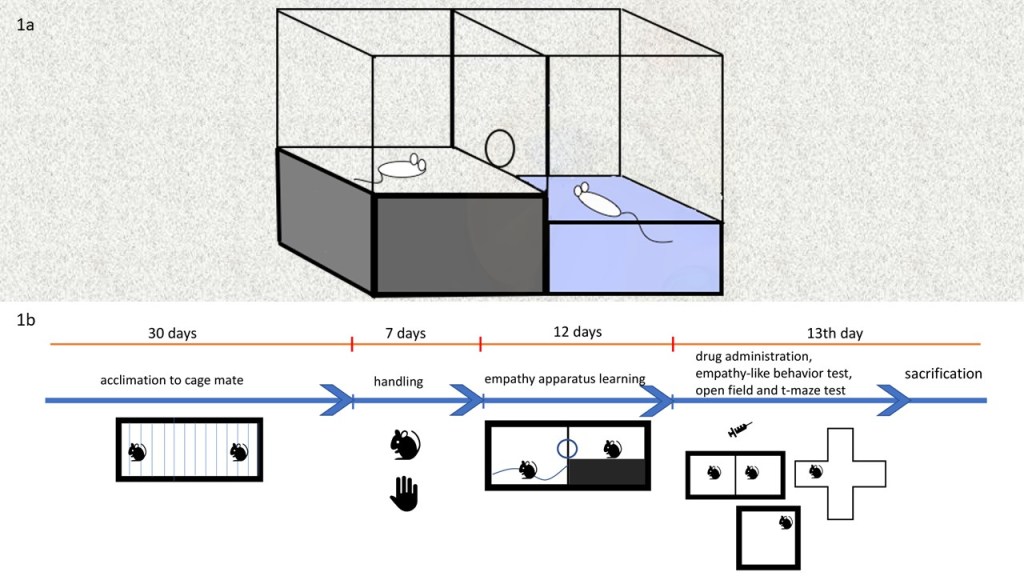
Figure 1. a The empathy-like behavior experiment. The rat rescues the cage mate by opening the door. b Experimental procedures.
RESULTS
Serum corticosterone levels were significantly different between two groups (t(14)=3,11, p=0.008). Compared to the CR group (2,25 ± 0,22 ng/ml), corticosterone levels were significantly decreased in the NTR group (1,95 ± 0,16 ng/ml) (Figure 2a). The door opening times were significantly different among the two groups (t(8,2)=4,2, p=0.003). When NTX-treated and non-NTX treated groups were compared, door opening latency in the NTR group (20,50±27,33 sec) were significantly decreased compared to door opening latency in the CR group (162,37±90,81 sec) (Figure 2b). The corticosterone levels were found to be moderately correlated with the door opening durations (r=0,525, p=0.03) (Figure 2c).
The open field test demonstrated a significant difference between the groups in terms of total distance traveled (t(14)=-5,35, p<0.0001). The total distance traveled measurements of the NTR group (1787 ± 340 cm) was significantly longer than CR group (1031 ± 209 cm) (Figure 3a). The spent time in the margin area showed no significant difference and in the centre area displayed significant difference between the groups (t(14)= -2,77, p=0,015) (Figure 3b, 3c) . In the centre area, spent time in the NTR group (6,26±2,79) was longer than in the CR group (3,19±1,39).
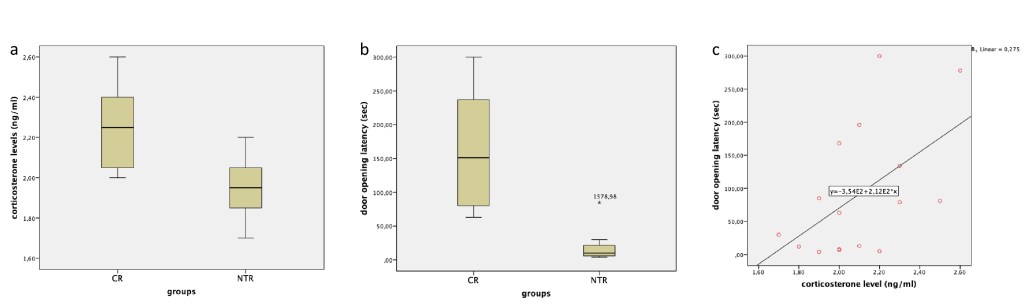
Figure 2. Serum corticosterone measurements, door opening durations and correlation analysis. a The serum corticosterone levels of the NTR group decreased compared to the CR group (p=0.008). b The door opening duration in the NTR group was faster than in the CR group (p=0.003). c There was a positive correlation between door opening times and corticosterone levels. NTX: Naltrexone, CR: Control.
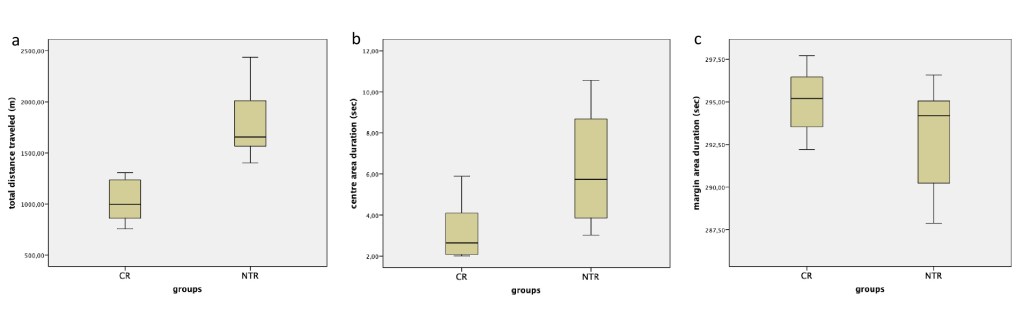
Figure 3. The open field test. a In the open field test, total distance traveled increased in the NTR group compared to the CR group (p<0.0001). b In the NTR group compared to the CR group, the time spent was long on the centre area (p=0.015). c On the margin area there was no significant difference between the groups. NTX: Naltrexone, CR: Control.
In the elevated plus maze test, spent time in the common area and closed arm showed no significant difference between the groups. The spent time in the open arm showed significant difference among the groups (t(14)= -3,14, p=0,007)(Figure 4 a-c). In the NTR group (8,15±3,76 sec), time spent in the open arm was long than in the CR group (3,21±2,35 sec). The entry frequency into open arms showed significant difference (t(14)= -2,55, p=0.023) and closed arms no significant difference among the groups. In the CR group, the frequency of entry unto the open arms (1,00±0,75) was less than in the NTR group (2,12±0,99) (Figure 4d, 4e).
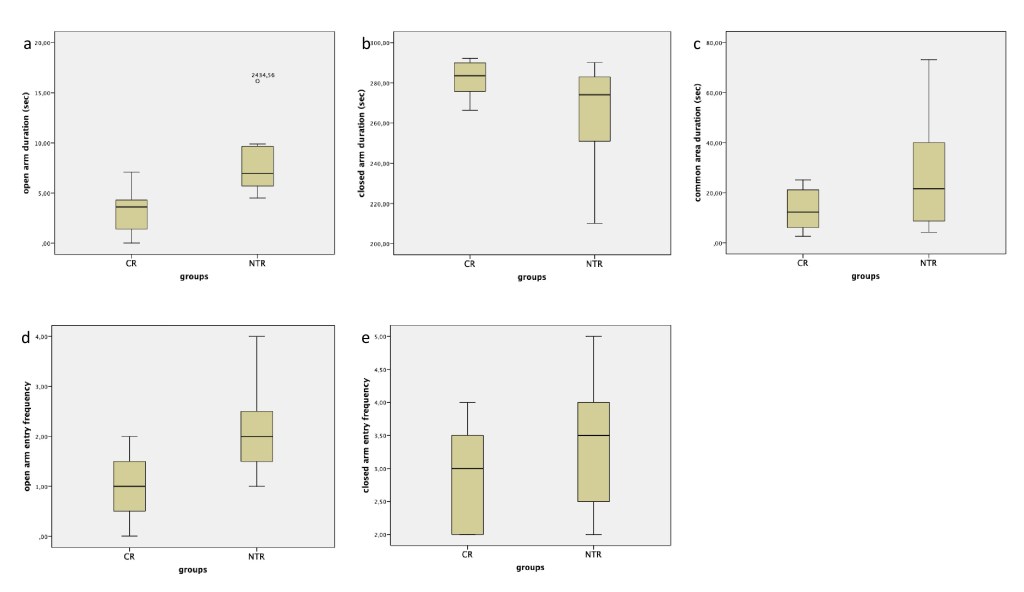
Figure 4. The elevated plus maze test. a The time spent in the open arm was long in the NTR group in comparison to the CR group (p=0.007). b-c On the closed arm and on the common area, the time spent showed no significant difference between groups. d The frequency of open arm entry was higher in the NTR group than CR group (p=0.023). e The frequency of closed arm entry displayed no significant difference between NTR and CR groups. NTX: Naltrexone, CR: Control.
DISCUSSION
We observed that compared to the CR group corticosterone levels were significantly decreased in the NTR group. In our study the anxiety tests of the CR and NTR groups exhibited changes consistent with corticosterone levels. In the open field test, the total distance traveled in the NTR group was longer and the time spent in the centre area was longer than in the CR group. In the elevated plus maze test, the NTR group spent more time on the open arm and enter into open arm more often than in the CR group. This consequences may be related to reduced anxiety level of NTR group. In rats, high anxiety causes hiding behavior towards an enclosed or edge area. In these tests, anxious rats exhibit immobility and freezing behaviour. When facing a threatening situation, the behavior inhibition system can be activated in the limbic system regions of the brain causing anxiety responses such as freezing and immobilization (24). Anxiolytic drugs increase locomotion and exploration in anxiety-like behavior tests (25). Previously, the anxiolytic efficacy of NTX was observed in rats exposed to prenatal stress (26). We consider these findings to be associated with decreased anxiety and increased locomotor activity in the NTR group.
We observed that the CR group did not open the door -or opened it later than the NTR group. The increased empathy-like behavior of the NTR group was associated with having lower anxiety levels than the CR group. It has been reported in animal experiments that a decrease in anxiety level increases social interaction (27). In our study, the correlation analysis showed that door opening durations and corticosterone levels displayed positive moderate correlation. Repression of anxiety in old rats may have reinforced social interaction and helping behavior.
NTX administered in the treatment of alcohol and opioid dependence is an opioid receptor blocker that can bind non-selectively to mu, delta and kappa receptors (28). In the anxiolytic activity of NTX against emotional stimuli, kappa opioid receptor (KOR) blocking is more effective than other receptors. It has been noted that NTX reduces emotional arousal against negative stimuli by possible kappa receptor antagonism (29).
During stress, the expression of dinorphins, one of the endogenous opioid peptides, increases and the dinorphins bind to KOR in the limbic system (30). Human and animal models have shown that dinorfin-KOR activation plays a role in negative emotional behavior responses such as stress-related dysphoria, aversive behavior, anxiety, and depression (30-33). For example, studies have demonstrated KOR antagonists disrupt the cycle of drug addiction by increasing the resistance to stress and resulting in avoiding negative emotions (34). Some studies suggest KOR antagonists as a therapeutic agent in stress-related disorders (35). Research has also reported that stress and aversive behavior induced by corticotropin-releasing factor can be prevented by KOR blocking (32). In rats, fear conditioning was found to increase the level of KOR in the amygdala, and when these receptors were blocked, conditioned fear and anxiety-like behaviors were reduced (36). In an another study in rats, KOR antagonism ameliorated CRF-induced deficit in attention-related performance testing (37). In our study, NTX may have caused anxiolytic efficacy by dinorfin-KOR blockage and may have improved the empathy-like behavior in old rats. On the other hand, NTX affects cognitive functions such as attention and perception (38). Enhanced cognitive functions may contribute to empathy-like behavior in the elderly (39).
As a result, we observed in this study the reinforcing effect of NTX on the empathy-like behavior of aged subjects. NTX, ameliorated empathy-like behavior with possible anxiolytic effect. Previously unknown effectiveness of NTX on the empathy-like behavior in elderly subjects is open to research.
Funding
This research received no external funding
Conflicts of interest
Authors declare that they have no conflict of interest.
Data availability
The data used in the present study are available from the corresponding author on reasonable request.
Author contributions
FH- design of the work; planning all behavioral and chemical analysis; the acquisition and analysis, interpretation of data; drafting the work; revising
CA, BK, RI- the acquisition and analysis, performing behavioral experiments; collecting data
SK, SK- the acquisition and analysis
MA, NU-design of the work; revising
Consent for publication
All authors reviewed the manuscript.
REFERENCES
- Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. Journal of cognitive neuroscience. 2007;19:42-58. doi: 10.1162/jocn.2007.19.1.42
- Hühnel I, Fölster M, Werheid K, Hess U. Empathic reactions of younger and older adults: No age related decline in affective responding. Journal of Experimental Social Psychology. 2014;50:136-143.
- Ruffman T, Henry JD, Livingstone V, Phillips LH. A meta-analytic review of emotion recognition and aging: implications for neuropsychological models of aging. Neuroscience and biobehavioral reviews. 2008;32:863-881. doi: 10.1016/j.neubiorev.2008.01.001
- Bailey PE, Henry JD, Von Hippel W. Empathy and social functioning in late adulthood. Aging & Mental Health. 2008;12:499-503.
- Frontiers in Neuroscience. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. Boca Raton (FL): CRC Press/Taylor & Francis Taylor & Francis Group, LLC.; 2007.
- Kandis S, Ates M, Kizildag S, Camsari GB, Yuce Z, Guvendi G, et al. Acetaminophen (paracetamol) affects empathy-like behavior in rats: Dose-response relationship. Pharmacol Biochem Behav. 2018;175:146-151.
- Yuksel O, Ates M, Kizildag S, Yuce Z, Koc B, Kandis S, et al. Regular Aerobic Voluntary Exercise Increased Oxytocin in Female Mice: Cause to Decrease Anxiety and Increase Empathy-Like Behaviors. Balkan medical journal. 2019.
- Karakilic A, Kizildag S, Kandis S, Guvendi G, Koc B, Camsari GB, et al. The effects of acute foot shock stress on empathy levels in rats. Behav Brain Res. 2018;349:31-36.
- Inagaki TK, Hazlett LI, Andreescu C. Opioids and social bonding: Effect of naltrexone on feelings of social connection and ventral striatum activity to close others. Journal of Experimental Psychology: General. 2019:No Pagination Specified-No Pagination Specified.
- Nummenmaa L, Tuominen L. Opioid system and human emotions. British journal of pharmacology. 2018;175:2737-2749.
- Danielli JF. Altruism and the internal reward system or the opium of the people. Journal of Social and Biological Structures. 1980;3:87-94.
- Jacobson ML, Browne CA, Lucki I. Kappa Opioid Receptor Antagonists as Potential Therapeutics for Stress-Related Disorders. Annual review of pharmacology and toxicology. 2020;60:615-636.
- Carr GV, Lucki I. Comparison of the kappa-opioid receptor antagonist DIPPA in tests of anxiety-like behavior between Wistar Kyoto and Sprague Dawley rats. Psychopharmacology. 2010;210:295-302.
- Turnes JM, Araya EI, Barroso AR, et al. Blockade of kappa opioid receptors reduces mechanical hyperalgesia and anxiety-like behavior in a rat model of trigeminal neuropathic pain. Behav Brain Res. 2022;417:113595. doi: 10.1016/j.bbr.2021.113595
- Jacobson ML, Wulf HA, Browne CA, Lucki I. The kappa opioid receptor antagonist aticaprant reverses behavioral effects from unpredictable chronic mild stress in male mice. Psychopharmacology. 2020;237:3715-3728.
- Reed B, Butelman ER, Kreek MJ. Kappa Opioid Receptor Antagonists as Potential Therapeutics for Mood and Substance Use Disorders. Handbook of experimental pharmacology. 2022;271:473-491.
- Rodefer JS, Nguyen TN. Naltrexone reverses age-induced cognitive deficits in rats. Neurobiology of Aging. 2008;29:309-313.
- Mouaffak F, Leite C, Hamzaoui S, Benyamina A, Laqueille X, Kebir O. Naltrexone in the Treatment of Broadly Defined Behavioral Addictions: A Review and Meta-Analysis of Randomized Controlled Trials. European Addiction Research. 2017;23:204-210.
- Moura F, Kildary M, Xavier F, Eliane F, Rodrigues L, Fabio M, et al. Behavioral, Neurochemical and Histological Changes in the Use of Low Doses of Naltrexone and Donepezil in the Treatment in Experimental Model of Alzheimer’s Disease by Induction of β-Amyloid1-42 in Rats. 2019:2518-0177.
- Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol. 2014;33:451-459. doi: 10.1007/s10067-014-2517-2
- Sato N, Tan L, Tate K, Okada M. Erratum to: Rats demonstrate helping behavior toward a soaked conspecific. Animal cognition. 2015;18:1049.
- Xia Y, Ye S, Shi J, Huang H. Relationship Between the Anxious Symptoms and the Neurotransmitter in Parkinson’s Mice with Different Dosages of MPTP %J Brazilian Archives of Biology and Technology. 2018;61.
- Rodrigues-Alves PdSeB, Flório JC, Lebrun I, Bernardi MM, Spinosa HdS. Moxidectin interference on motor activity of rats %J Brazilian Archives of Biology and Technology. 2009;52:883-891.
- Steimer T. Animal models of anxiety disorders in rats and mice: some conceptual issues. Dialogues Clin Neurosci. 2011;13:495-506.
- Tucker LB, McCabe JT. Measuring Anxiety-Like Behaviors in Rodent Models of Traumatic Brain Injury. Frontiers in behavioral neuroscience. 2021;15:682935.
- Keshet GI, Weinstock M. Maternal naltrexone prevents morphological and behavioral alterations induced in rats by prenatal stress. Pharmacol Biochem Behav. 1995;50:413-419.
- File SE, Lippa AS, Beer B, Lippa MT. Animal tests of anxiety. Current protocols in neuroscience. 2004;Chapter 8:Unit 8.3.
- Littleton J, Zieglgänsberger W. Pharmacological Mechanisms of Naltrexone and Acamprosate in the Prevention of Relapse in Alcohol Dependence. 2003;12:s3-s11.
- Wardle MC, Bershad AK, de Wit H. Naltrexone alters the processing of social and emotional stimuli in healthy adults. Soc Neurosci. 2016;11:579-591.
- Knoll AT, Carlezon WA, Jr. Dynorphin, stress, and depression. Brain Res. 2010;1314:56-73.
- Corder G, Castro DC, Bruchas MR, Scherrer G. Endogenous and Exogenous Opioids in Pain. Annu Rev Neurosci. 2018;41:453-73.
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:407-414.
- Qi C-t, Zou H, Zhang C-h, Xie Q-l, Jin M-l, Yu L. Effect of GNTI, a kappa opioid receptor antagonist, on MK-801-induced hyperlocomotion and stereotypy in mice. Acta Pharmacologica Sinica. 2006;27:1401-1408.
- Chavkin C, Koob GF. Dynorphin, Dysphoria, and Dependence: the Stress of Addiction. Neuropsychopharmacology. 2016;41:373-374. doi: 10.1038/npp.2015.258
- Van’t Veer A, Carlezon WA, Jr. Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology. 2013;229:435-452.
- Knoll AT, Muschamp JW, Sillivan SE, et al. Kappa opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biol Psychiatry. 2011;70:425-433. doi: 10.1016/j.biopsych.2011.03.017
- Van’t Veer A, Yano JM, Carroll FI, Cohen BM, Carlezon WA, Jr. Corticotropin-releasing factor (CRF)-induced disruption of attention in rats is blocked by the κ-opioid receptor antagonist JDTic. Neuropsychopharmacology. 2012;37:2809-2816. doi: 10.1038/npp.2012.151
- Lim AC, Grodin EN, Green R, et al. Executive function moderates naltrexone effects on methamphetamine-induced craving and subjective responses. The American journal of drug and alcohol abuse. 2020;46:565-576. doi: 10.1080/00952990.2020.1741002
- Goodhew SC, Edwards M. The relationship between cognitive failures and empathy. Personality and Individual Differences. 2022;186:111384.
